Product Pipeline
SB0101 – A Novel Non-Opioid Biologic for Neuropathic Pain
Sciatica and painful diabetic neuropathy (PDN) affect approximately 40 million patients in EU and US. Currently there is no registered drug for sciatica and around 50% of patients with PDN are inadequately treated.
With its unique and reparative mechanism, SB0101 is poised to become a first-in-class therapeutic and has demonstrated clinically meaningful effects in both a phase I multiascending dose study and in a multicenter phase IIa proof-of-concept study in chronic sciatica. Encouraged by these positive clinical data we are ready to initiate phase IIb trials, IND approved by the FDA.
SB0201 – A Novel Reparative Gene Therapy Device for Treatment of Sensorineural Hearing Loss
More than 35 million patients suffer from sensorineural hearing loss (SNHL), a condition frequently impacting people’s ability to socialize, work and to live normal lives. SNHL develops in response to damage of the sensory hair cells and nerve pathways of the inner ear that sense and transmit sound to the brain. Building on the direct implantation in the cochlea, we have developed the gene therapy device SB0201 that complements and expands the therapeutic scope for many patients currently with suboptimal treatment options. Encouraged by the therapeutic effects obtained in preclinical models SB0201 has entered preclinical safety studies to prepare for clinical translation.

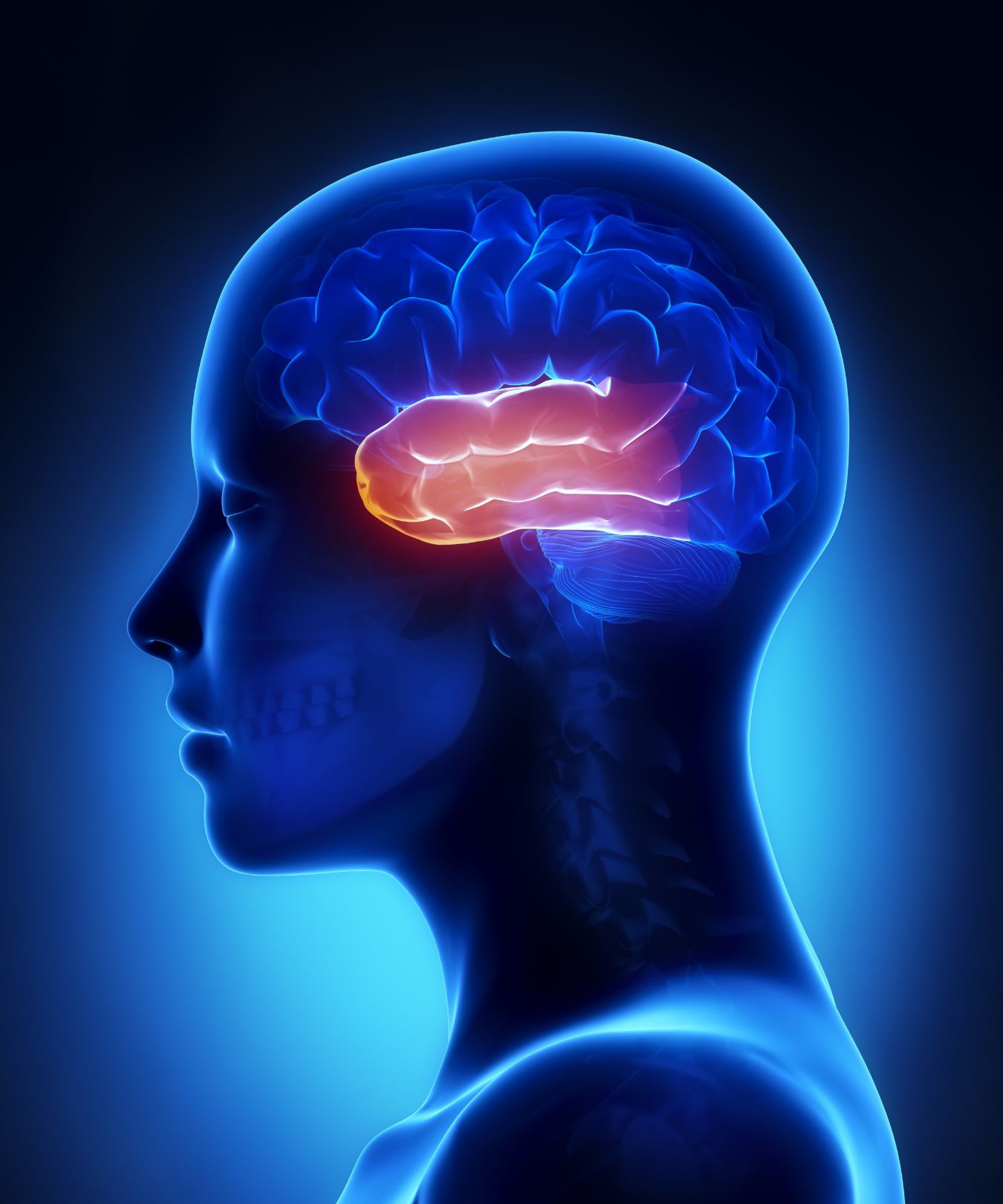
SB0501 – A first in class reparative therapy for pharmacoresistant temporal lobe epilepsy (TLE)
Epilepsy affects tens of millions of individuals worldwide and is characterized by debilitating seizures, cognitive decline, and psychiatric disorders. A significant portion of epilepsies in adults originate focally from temporal lobe structures. These TLEs cannot be cured and one third of all patients do not respond to pharmacological treatment. Even when effective at reducing seizures, the currently available drugs typically cause significant unwanted side effects. Because of the focal nature of TLE, we have developed the gene therapy device SB0501 that targets the affected anatomical structures and secretes potent antiepileptic biologics. SB0501 has shown strong antiepileptic effects in animal models of TLE and are associated with cognitive improvements and no evidence of side effects.
Precision therapies in research phase
Frontotemporal dementia (FTD) and Parkinson’s disease (PD) are heterogeneous neurodegenerative disorders in terms of clinical expression and disease pathology. It is therefore unlikely that there will be a one-therapy-fix all solution. Rather, tailored therapies for specific disease subtypes are more likely to succeed.

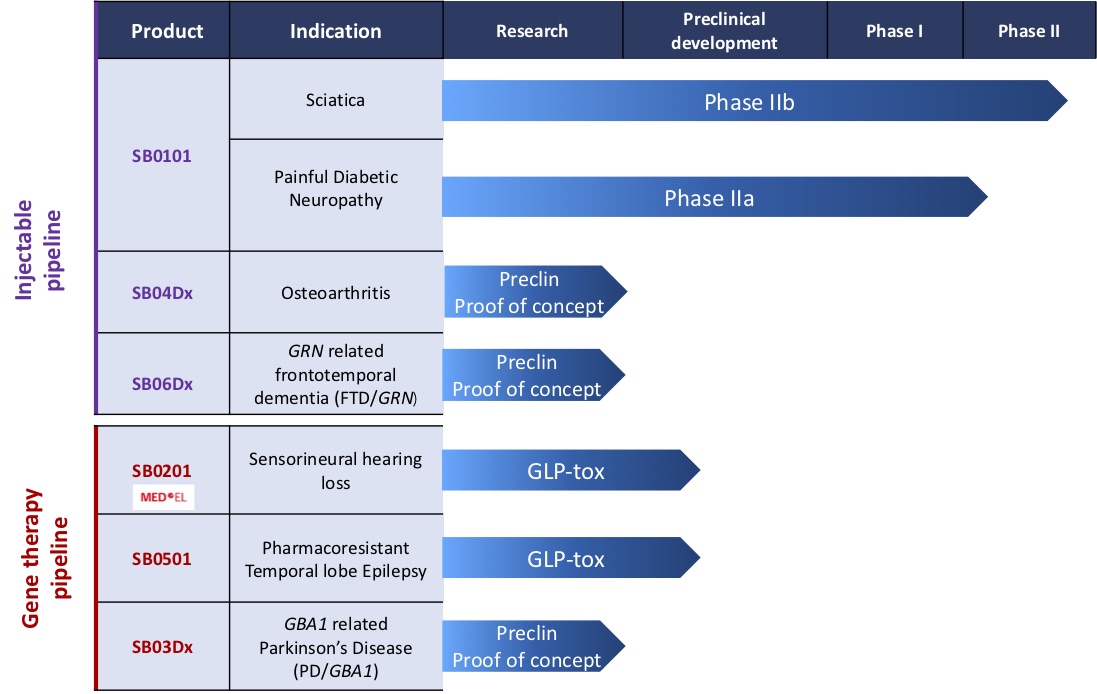
Product
Indication
Research Preclin. dev.
Phase I Phase II
SB04Dx
SB06Dx
Injectable pipeline
Osteoarthritis
GRN related frontotemporal dementia (FTD/GRN)
Preclinical (Proof of concept)
SB0201
SB0501
SB03Dx
Gene therapy pipeline
Sensorineural hearing loss
Pharmacoresistent temporal lobe epilepsy
GBA1 related Parkinsons Disease (PD/GBA1)
GLP-tox
GLP-tox
Preclinical (Proof of concept)